The reason why lead batteries dissolve sulfation and revive with “LIVagain”.
Table of contents
Deterioration of lead-acid batteries due to sulfation.
Why does “LIVagain” dissolve sulfation (lead sulfate)?
First, please see the experiment in which sulfation (lead sulfate) dissolves.

1.Measure the specific gravity value. (reference value)
2.Inject “LIVagain”. (20cc each cell)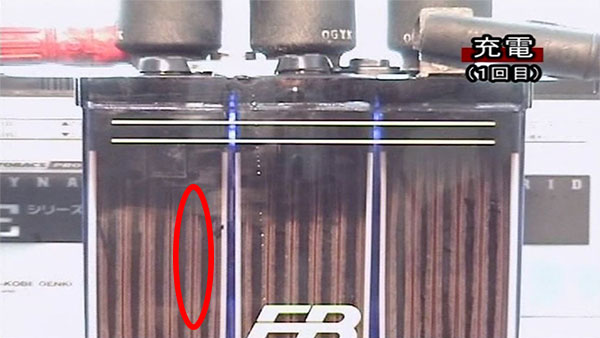
3. Charging. (1st time)
Sulfation adheres to the electrode plate.
4.Measure the specific gravity value. (second time)
5.Discharge to final voltage.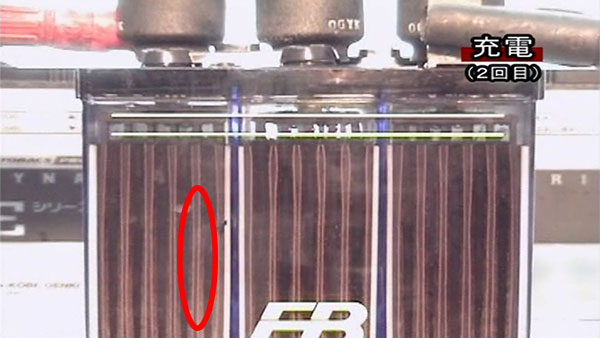
6.Charging. (2nd charge)
The sulfation attached to the electrode during the first charge has melted considerably.
7.Measure the specific gravity value. (3rd time)
8.Discharge to final voltage.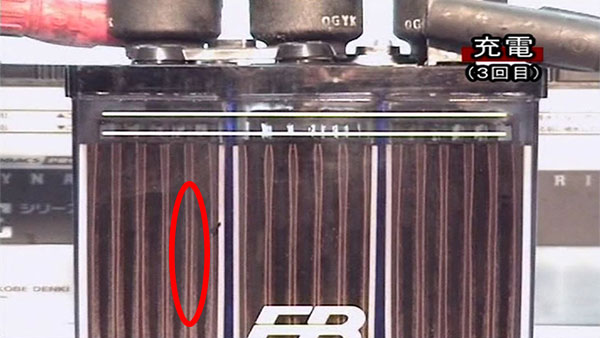
9.Charge (3rd time)
The attached sulfation is thinner than when charging for the second time.
10.Measure specific gravity. (4th time)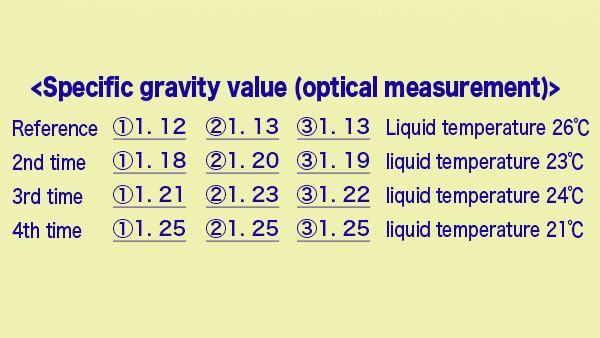
11.Specific gravity value. (Optical measurement)
- material provider
- Ion Science Co., Ltd.
Most of the lead battery revival agents so far were "battery enhancers (additives that only enhance)".
Batteries (lead-acid batteries) use lead for the electrode plates, and the dilute liquid sulfuric acid and lead cause a chemical reaction to generate electricity. 75% of lead battery deterioration is caused by white sulfate lead (sulfation) generated in this process (diluted sulfuric acid turns into electricity).
The crystallized lead sulfate at this time adheres to the electrode plate over time and covers the entire electrode plate and becomes an insulator, shortening the life.
It seems that some battery revival agents forcefully peel off the entire surface of the electrode plate. This will damage the plate, which is an important material that generates electricity.
The important thing is to "dissolve" the generated sulfation = lead sulfate crystals in the original dilute sulfuric acid and dissolve it back to its original state.
Preventing sulfation means not generating electricity, so the life of the battery cannot be extended unless there is an effect to return it to its original state over time after sulfation occurs. Electricity is generated when lead and sulfuric acid react and chemically change, releasing electrons and generating electricity. What is important is how to finely crystallize + ions of lead and - ions of sulfuric acid in the process of generating electricity.
“LIVagain” is the third ion solvating electron. It reduces ion electrons and dissolves battery sulfation.
However, it cannot be used for batteries in the following conditions.
- Battery voltage is less than 8V
- Battery fluid turns brown
- Battery with damaged battery plates due to overcharging
- Over discharge due to long-term storage
- Maintenance-free type
- The composition of the battery fluid is different from usual due to other companies' battery strengthening agents (germanium-based, carbon-based, etc.).
How to use
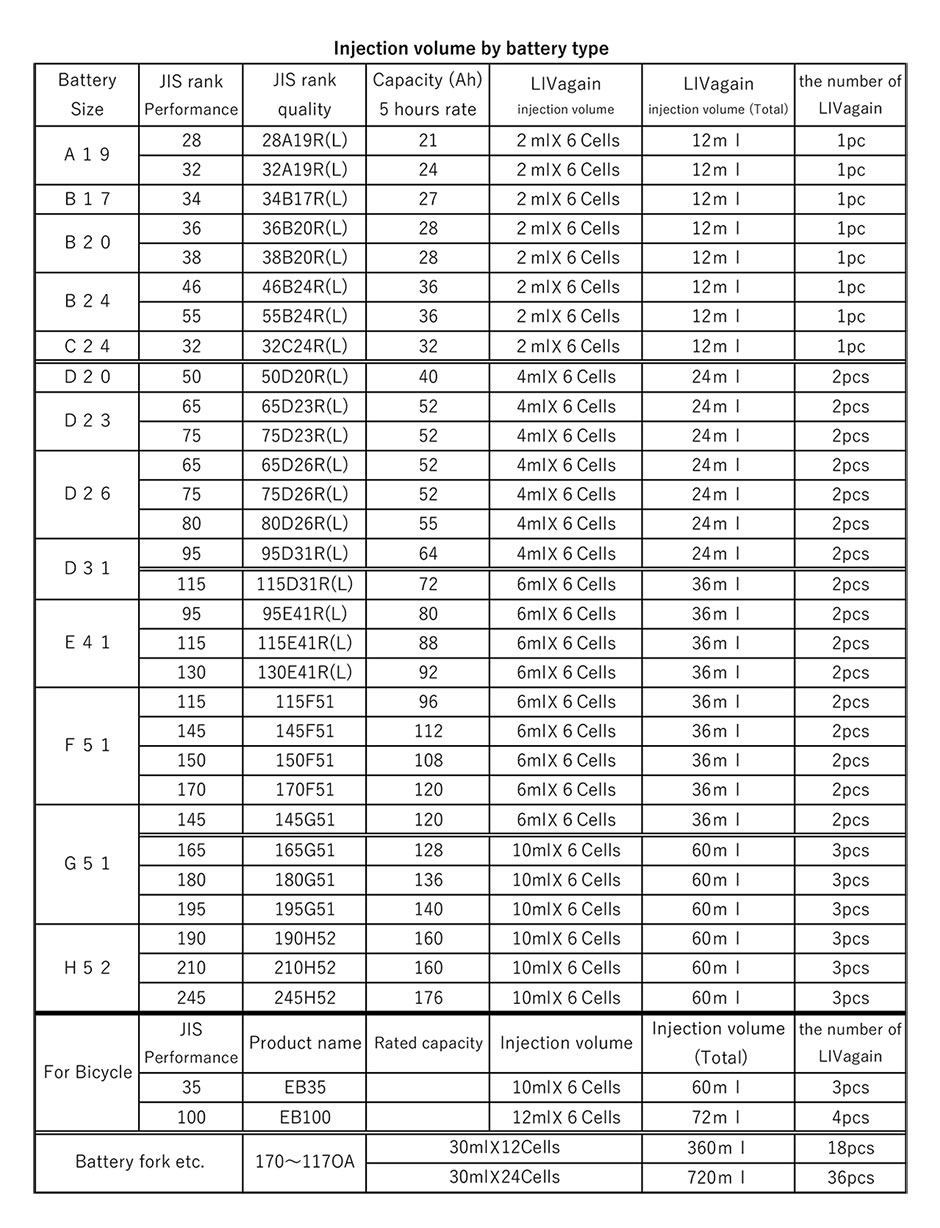
- Please prepare the injection amount of “LIVagain” referring to the injection amount table.
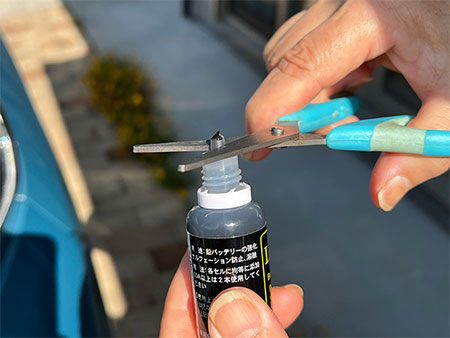
- Cut the inner plug of “LIVagain” with scissors.
- Remove the battery cell lid and inject the appropriate amount. Be careful not to press the “LIVagain” container too hard, as the inner stopper may come off and fall off. You can use it with the inner plug removed.

How is it?
Use “LIVagain” to revive your car battery.
For home use, no special equipment or knowledge is required. “LIVagain” can dissolve sulfation, and extend the life of lead-acid batteries.
“LIVagain” is a product that has been provided to companies for more than 10 years for commercial use and has been repackaged for home use.
Click here for materials when using “LIVagain” for commercial forklifts, etc. (Japanese only)




